
Robert G. Cooper is one of the most influential innovation thought-leaders in the business world today. He pioneered many ground-breaking discoveries in product innovation, including the Stage-Gate® Idea-to-Launch Process, now implemented by almost 80% of North American companies. Having spent 40+ years studying the practices and pitfalls of 3000+ new product projects in thousands of companies, he has assembled the world’s most comprehensive research in the field of product innovation management.
For more information on the Stage-Gate® process and its founders, visit:
www.stage-gate.com/our-founders.

Users of Stage-Gate® process tools will significantly reduce the risks associated with a new product launch. The process has been proven to:

The following seven aspects of the Stage-Gate® process are considered the most critical for success in the developmental process at Empower Medical Devices.
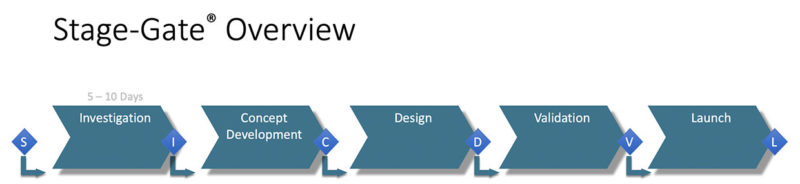
The process model adopted at Empower Medical Devices is a direct take-off of the Stage-Gate® Idea-to-Launch process model.

Critical to the Stage-Gate® process, gatekeepers are a decision-making group comprised of company executives and managers, and the medical professional/inventor.
Gatekeepers must:

A formal Gate Review is conducted after each step in the process, with the medical professional included – this is a key decision point involving the doctor. If the project passes the review, we decide to move onto the next step, determine time frames, actions required, etc.
The Gatekeepers’ function is to make decisions.
Some of the procedures followed:
Let’s start the conversation today!
Phone: 317-690-0764
© 2024 Empower Medical Devices LLC. All rights reserved.